Iso 13485 Software Validation Template - A suggested layout of documenting risk within the master validation plan; Web record of software validation [iso 13485 templates] iso 13485 document template: Validation of software used in manufacturing. Designed with your company in mind. Web you can buy the iso 13485 standard here. Document templates contain an average of twenty comments each, and offer clear guidance for filling them out. Web download this free checklist to see which mandatory documentation is required by iso 13485. Web the documentation template may be used for iso 13485 certification audit purposes. Web templates iso 13485 templates updated june 9, 2022 template: Does it do what it says on the tin?
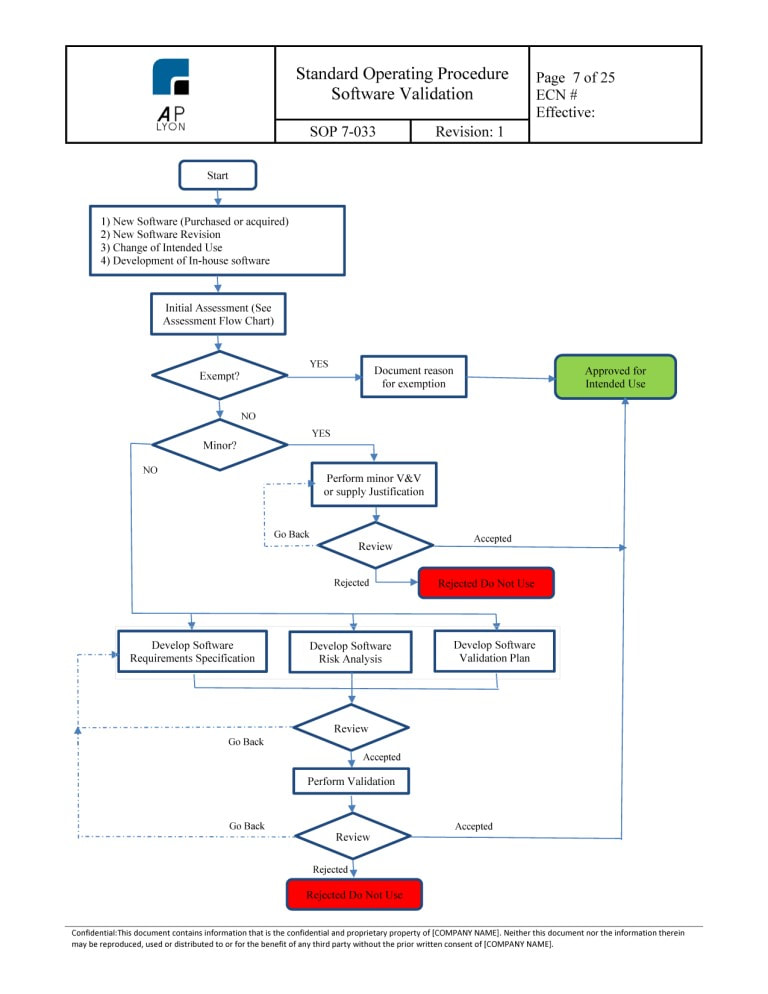
Software Validation Risk Assessment Template Master of
The intended purpose is achieved, validation. Web templates iso 13485 templates updated january 19, 2023 template: Like our facebook page and comment here or. Web templates iso 13485 templates updated june 9, 2022 template: Designed with your company in.

Iso 13485 Software Validation Template PDF Template
Web templates iso 13485 templates updated june 9, 2022 template: Web download this free checklist to see which mandatory documentation is required by iso 13485. So which software does this include? Mapping of requirements to documents sven piechottka template download this is a free template, provided by openregulatory. Web templates iso 13485 templates updated june 9, 2022 template:

Iso 13485 & 21 CFR 820 Template Documentation Operational Procedure Qop
Web an iso 13485 audit checklist is utilized by quality managers to determine if the organization’s qms is aligned with the iso 13485:2016 standard. Web you can buy the iso 13485 standard here. Quality 3.6.2 (bs en iso 9001:2015) Web iso 13485:2016 section 4.1.6 “quality management system, general requirements” and 7.5.6 “validation of processes for production and service provision” state.

ISO 13485 software validation process
Editable ms word and ms excel policies, procedures, plans, and forms that you can adapt to your company needs. Examples of computer software used in the quality management system; Web the iso 13485 is the standard for quality management in the medical device industry. Web how to meet the software validation requirements of iso 13485:2016; Does it do what it.

Software Validation Template Iso 13485
Activities should be proportionate to risk. With safetyculture (formerly iauditor), quality managers can: Web templates iso 13485 templates updated june 9, 2022 template: Designed with your company in. The documentation template may be used for iso 13485 certification audit purposes.

Software Validation Template Iso 13485
You can buy the iso 13485 standard here. Document templates contain an average of twenty comments each, and offer clear guidance for filling them out. Like our facebook page and comment here or. Web validation of computer software is specified in section 4.1.6 of iso 13485:2016. Designed with your company in.

Software Validation Procedure
Activities should be proportionate to risk. Like our facebook page and comment here or. Web you can buy the iso 13485 standard here. Web free iso 13485 software validation template. Record of software validation the record provides information about software validation results.
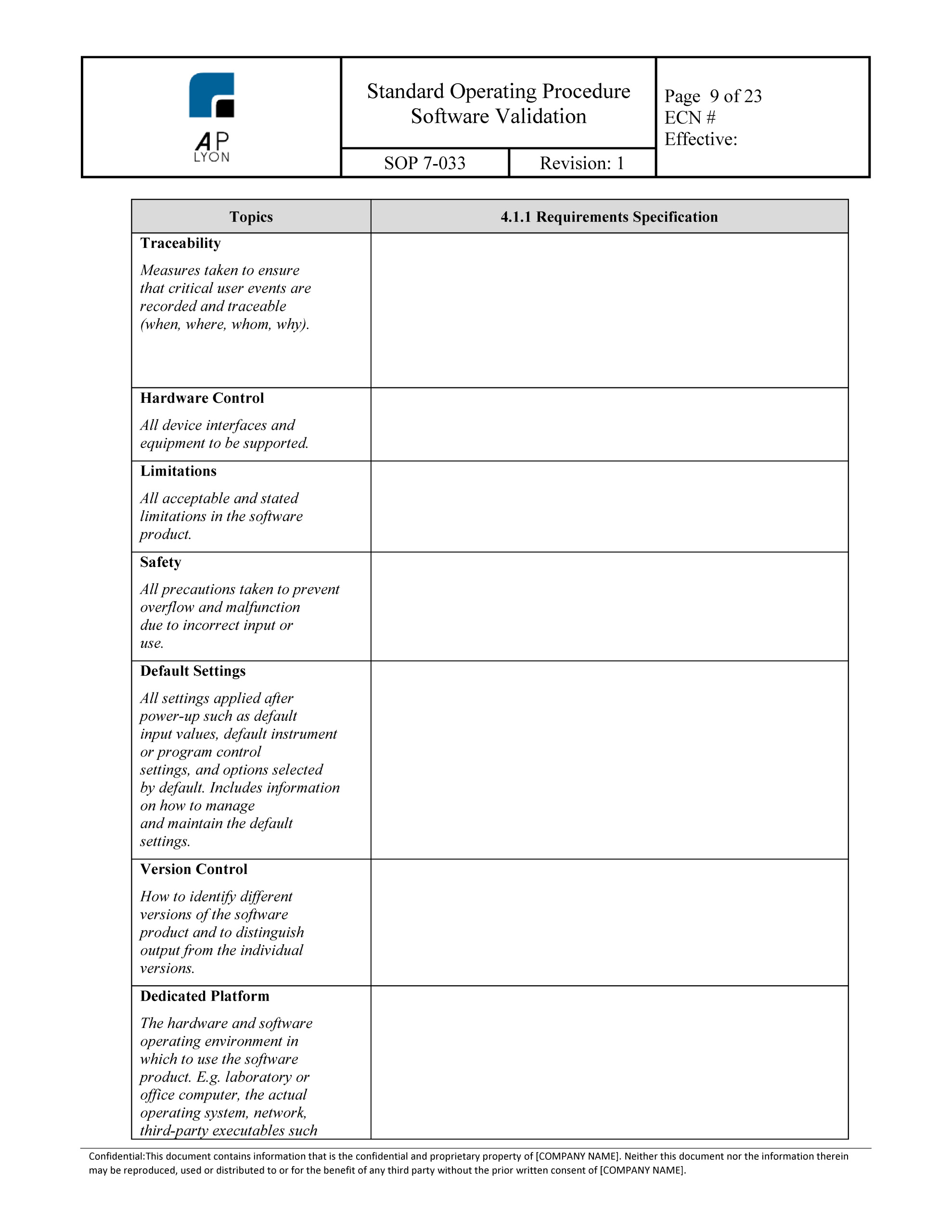
Software Validation Template
Web the documentation template may be used for iso 13485 certification audit purposes. Web templates iso 13485 templates updated august 31, 2022 template: So which software does this include? This procedure will help guide your company to properly evaluating all qms software throughout its lifecycle. Device validation and verification is critical to successful manufacturing and compliance with iso 13485.
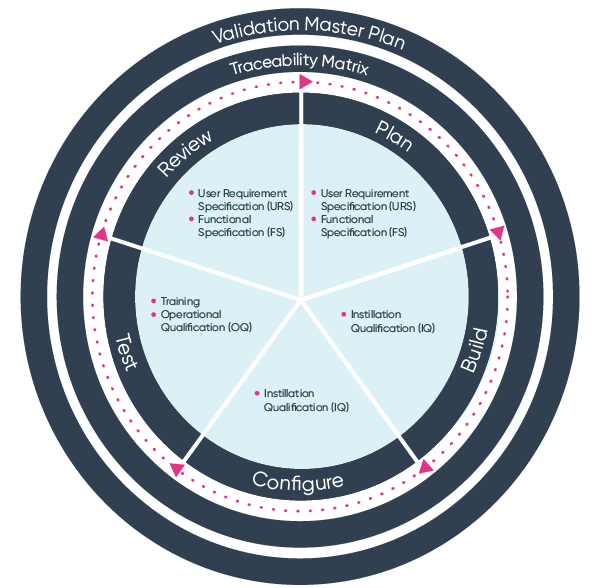
ISO 13485 software validation process Ideagen
Validate software which is used in the quality management system prior to use and after changes. Web templates iso 13485 templates updated august 31, 2022 template: Email us here from your work email (verifiable domain from company website)* to receive a free copy of this sop free of charge! With safetyculture (formerly iauditor), quality managers can: Record of software validation.

Free ISO 13485 Process Validation Template
Here are all our posts on this standard, and also all questions our consulting clients have asked us about the iso 13485 so far. Email us here from your work email (verifiable domain from company website)* to receive a free copy of this sop free of charge! How to categorise the software used at your medical device company; The documentation.
Web the documentation template may be used for iso 13485 certification audit purposes. How to categorise the software used at your medical device company; Document templates contain an average of twenty comments each, and offer clear guidance for filling them out. Web validation 3.8.13 (bs en iso 9001:2015) confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been fulfilled. Web iso 13485:2016 section 4.1.6 “quality management system, general requirements” and 7.5.6 “validation of processes for production and service provision” state the following “the organisation. You can buy the iso 13485 standard here. This procedure will help guide your company to properly evaluating all qms software throughout its lifecycle. Examples of computer software used in the quality management system; The main messages there are: Web templates iso 13485 templates updated january 19, 2023 template: Here are all our posts on this standard, and also all questions our consulting clients have asked us about the iso 13485 so far. Use the iso 13485 standard to ensure recognition and regulatory compliance. Sop software validation sven piechottka template download this is a free template, provided by openregulatory. Email us here from your work email (verifiable domain from company website)* to receive a free copy of this sop free of charge! Like and subscribe us on youtube and comment here. Software validation form sven piechottka template download this is a free template, provided by openregulatory. Designed with your company in. Designed with your company in mind. Web how to meet the software validation requirements of iso 13485:2016; This procedure is intended to meet the requirements of iso 13485:2016, clause 7.3.6 and 7.3.7 for design verification and design validation of medical device products.