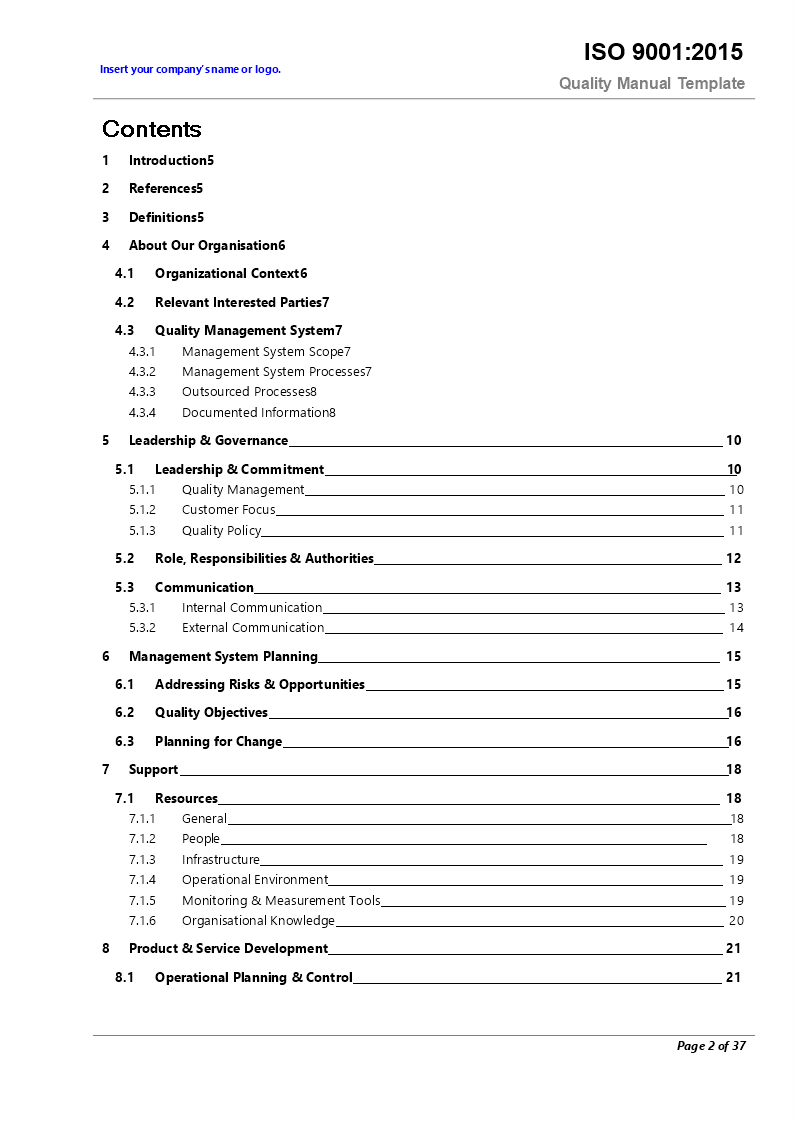
Iso 17025 Quality Manual Template - The management system is outlined in section 8. Web the perfect iso 17025 quality manual template for busy labs. Web this quality system document (qsd) defines or identifies the policies, procedures and requirements of organizations that choose to comply with the requirements of iso 17025 as a calibration laboratory. This system has been used by thousands of laboratories over the past 20 years to achieve accreditation. It is intended to aid laboratories to conform to the iso/iec 17025 requirements as well as when they transition to the revised 2017 standard. The premium template is offered as a microsoft word document. A new version of the standard was published by iso and the international electrotechnical commission ( iec) in. Create a custom quality management system in less than 30 days. The layout, format, and content is guaranteed to save time. Any local documents, procedures and policies associated with iso 17025 compliance for calibration laboratories must

Iso 17025 Quality Manual Template Free energyevery
For the introduction of the iso 17025 standard, you need: Any local documents, procedures and policies associated with iso 17025 compliance for calibration laboratories must Quality assurance procedure the purpose of this procedure is to detail the activities required to ensure that the organization maintains a quality assurance system that ensures valid. Web we provide iso 17025 implementation packages which.
Iso 17025 Quality Manual Template Free Pdf FREE PRINTABLE TEMPLATES
Web the perfect iso 17025 quality manual template for busy labs. The iso 17025 quality manual template is an efficient system to write your laboratory quality management documentation for laboratory accreditation to the iso/iec 17025:2017 standard. The quality manual, based on iso 17025, is used to document the lab quality management system of an organization. The quality policy statement is.

ISO/IEC 170252017 Quality Manual Template isobudgets
The need to gain iso 17025 compliance and accreditation impacts laboratories of all types and sizes. Management requirements and technical requirements. Web iso 17025:2017 documentation kit for environment / chemical testing laboratory. Check out the iso 17025:2017 quality manual template free preview. The quality manual, based on iso 17025, is used to document the lab quality management system of an.

Iso 17025 Standard Operating Procedure Template
These editable iso 17025 documents address all the elements of laboratory iso 17025 2017 accreditation. Web iso 17025 document template: The iso 17025 quality manual template can be applied to any type or. The quality manual, based on iso 17025, is used to document the lab quality management system of an organization. The layout, format, and content is guaranteed to.

ISO/IEC 170252017 Quality Manual Template isobudgets
The layout, format, and content is guaranteed to save time. The quality policy statement is detailed in section 8.2.2. Web buy the iso 17025:2017 quality manual template or iso 17025:2017 management system template that includes the measurement uncertainty calculator, forms, procedures. Executive summary of the iso 17025 quality manual template package: Web iso 17025 document template:

Iso 9001 Quality Manual Template Free Download FREE PRINTABLE TEMPLATES
Web iso17025 quality manual template provides laboratories throughout the world with a package to get iso 17025 accreditation. Iso/iec 17025:2017 accreditation implementation package with quality manual, procedures and forms Get ready for iso 17025 accreditation in 30 days or less with this complete quality manual template. Web iso 17025:2017 documentation kit for environment / chemical testing laboratory. Web buy the.
Iso 17025 Quality Manual Template Free bobele
Management requirements are related to the operation and effectiveness of the quality management system within the laboratory and has similar requirements to iso 9001. The document style is consistent with the styles used throughout all documents, forms and templates. 1 quality manual, 12 procedures, 13 lists, and 18 forms add to cart instant download download this item immediately after purchase..
Iso 17025 Quality Manual Template Free britishcelestial
Executive summary of the iso 17025 quality manual template package: For the introduction of the iso 17025 standard, you need: The document style is consistent with the styles used throughout all documents, forms and templates. Management requirements are related to the operation and effectiveness of the quality management system within the laboratory and has similar requirements to iso 9001. Quality.
Iso 17025 Quality Manual Template Free
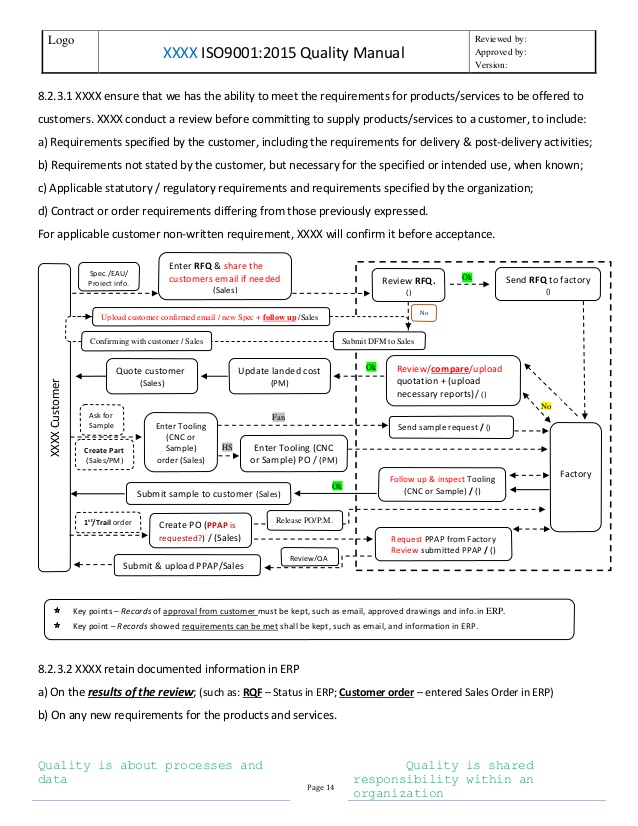
Web this publication provides practical guidance to iso/iec 17025, the international standard for the competence of testing and calibration laboratories. The quality policy statement is detailed in section 8.2.2. This comprehensive package allows the user to develop an iso 17025: Web the following management system procedures are included in our iso 17025 quality manual template package: Web the iso 17025.

Iso 17025 Quality Manual Template Free Pdf
Web we provide iso 17025 implementation packages which help in writing the required documentation and assist with implementation of the accreditation requirements. Create a custom quality management system in less than 30 days. The iso 17025 quality manual template is an efficient system to write your laboratory quality management documentation for laboratory accreditation to the iso/iec 17025:2017 standard. Don’t spend.
Check out the iso 17025:2017 quality manual template free preview. The premium template is offered as a microsoft word document. Web the following management system procedures are included in our iso 17025 quality manual template package: Web the iso 17025 quality manual template provides laboratories from around the world with a proven implementation package to attain accreditation through their accreditation body. Web the iso 17025 quality manual template is the most efficient approach for you to implement your iso 17025 based quality management system. Web this publication provides practical guidance to iso/iec 17025, the international standard for the competence of testing and calibration laboratories. The quality policy statement is detailed in section 8.2.2. Get ready for iso 17025 accreditation in 30 days or less with this complete quality manual template. This system has been used by thousands of laboratories over the past 20 years to achieve accreditation. In addition to describing the organization’s structure, the manual states the general requirements of impartiality and confidentiality and other specific requirements for structure, resource, process and. Web iso 17025:2017 documentation kit for environment / chemical testing laboratory. This comprehensive package allows the user to develop an iso 17025: Web personnel performing specific tasks within the management system that may directly or indirectly affect the conformance of the product to quality requirements are qualified on the basis of appropriate education, qualification, training, technical knowledge, experience, and/or demonstrated skills, as required. Management requirements are related to the operation and effectiveness of the quality management system within the laboratory and has similar requirements to iso 9001. The iso 17025 quality manual template is an efficient system to write your laboratory quality management documentation for laboratory accreditation to the iso/iec 17025:2017 standard. The range of laboratory activities is addressed in section 5.3. Any local documents, procedures and policies associated with iso 17025 compliance for calibration laboratories must The management system is outlined in section 8. Why pay them to fill in the blanks for you? Create a custom quality management system in less than 30 days.